目的 探讨胶质瘤中调节性T细胞浸润及IL-6、TGF-β1表达水平及与病情复发的相关性。方法 收集胶质瘤患者24例,初发时在南阳市第二人民医院行手术切除,且术后病情复发,在南阳市第二人民医院行2次手术治疗。取2次术后组织标本共48份,分为初发组、复发组。另取同期脑外伤减压手术切除的正常脑组织标本6份为对照组。免疫组化染色检测初发组、复发组T细胞浸润及IL-6、TGF-β1表达水平;酶联免疫吸附法检测初发组、复发组、对照组的组织匀浆中IL-6、TGF-β1浓度。结果 24例胶质瘤患者病情复发后病理级别较初发时明显增加,其中复发组Ⅲ级和Ⅳ级患者比例明显高于初发组,Ⅰ级和Ⅱ级患者比例明显低于初发组,差异有统计学意义(P<0.05)。调节性T细胞在胶质瘤组织中呈散在浸润,随病理分级的增加,调节性T细胞阳性率明显提升,差异有统计学意义(P<0.01);其中复发组调节性T细胞阳性率明显高于初发组,差异有统计学意义(P<0.01)。IL-6、TGF-β1在胶质瘤组织中呈团簇状分布,随病理分级增加,IL-6、TGF-β1阳性率均明显提升,差异有统计学意义(P<0.01);其中复发组IL-6、TGF-β1阳性率明显高于初发组,差异有统计学意义(P<0.05)。初发组与复发组胶质瘤组织匀浆中IL-6、TGF-β1浓度明显高于对照组,复发组IL-6、TGF-β1浓度明显高于初发组,差异有统计学意义(P<0.01)。结论 胶质瘤微环境中调节性T细胞浸润及IL-6、TGF-β1表达增加,且随恶性程度的增加及胶质瘤复发明显增高,可作为评价胶质瘤进展和预后的免疫指标。
胶质瘤中调节性T细胞浸润及IL-6 TGF-β1表达与病情复发的相关性
王 栋1) 白雪蕾2) 吴环立1)△
南阳市第二人民医院 1)神经外科一病区 2)心脏生理实验室,河南 南阳 473012
作者简介:王栋,Email:813944169@qq.com
△通信作者:吴环立,Email:813944169@qq.com
【摘要】 目的 探讨胶质瘤中调节性T细胞浸润及IL-6、TGF-β1表达水平及与病情复发的相关性。方法 收集胶质瘤患者24例,初发时在南阳市第二人民医院行手术切除,且术后病情复发,在南阳市第二人民医院行2次手术治疗。取2次术后组织标本共48份,分为初发组、复发组。另取同期脑外伤减压手术切除的正常脑组织标本6份为对照组。免疫组化染色检测初发组、复发组T细胞浸润及IL-6、TGF-β1表达水平;酶联免疫吸附法检测初发组、复发组、对照组的组织匀浆中IL-6、TGF-β1浓度。结果 24例胶质瘤患者病情复发后病理级别较初发时明显增加,其中复发组Ⅲ级和Ⅳ级患者比例明显高于初发组,Ⅰ级和Ⅱ级患者比例明显低于初发组,差异有统计学意义(P<0.05)。调节性T细胞在胶质瘤组织中呈散在浸润,随病理分级的增加,调节性T细胞阳性率明显提升,差异有统计学意义(P<0.01);其中复发组调节性T细胞阳性率明显高于初发组,差异有统计学意义(P<0.01)。IL-6、TGF-β1在胶质瘤组织中呈团簇状分布,随病理分级增加,IL-6、TGF-β1阳性率均明显提升,差异有统计学意义(P<0.01);其中复发组IL-6、TGF-β1阳性率明显高于初发组,差异有统计学意义(P<0.05)。初发组与复发组胶质瘤组织匀浆中IL-6、TGF-β1浓度明显高于对照组,复发组IL-6、TGF-β1浓度明显高于初发组,差异有统计学意义(P<0.01)。结论 胶质瘤微环境中调节性T细胞浸润及IL-6、TGF-β1表达增加,且随恶性程度的增加及胶质瘤复发明显增高,可作为评价胶质瘤进展和预后的免疫指标。
【关键词】 胶质瘤;调节性T细胞;IL-6;TGF-β1;表达变化;病情复发
【中图分类号】 R730.264 【文献标识码】 A 【文章编号】 1673-5110(2019)01-0017-06 DOI:10.12083/SYSJ.2019.01.004
Correlation between regulatory T cell infiltration and IL-6 TGF-β1 expression in gliomas and relapse
WANG Dong1),BAI Xuelei2),WU Huanli1)
Second People's Hospital of Nanyang City 1) Department of Neurosurgery,2) Heart Physiology Laboratory,Nanyang 473012,China
【Abstract】 Objective To investigate the relationship between regulatory T cell infiltration and the expression of IL-6 and TGF-β1 in gliomas and their recurrence.Methods Twenty-four patients with glioma were collected and surgically resected at the Second People's Hospital of Nanyang City at the initial stage.The patients relapsed after surgery and were treated twice in the Second People's Hospital of Nanyang City.A total of 48 postoperative tissue specimens were taken and divided into the initial group and the recurrence group.In addition,6 specimens of normal brain tissue removed from the decompression of brain trauma in the same period were taken as the control group.Immunohistochemical staining was used to detect T cell infiltration and IL-6 and TGF-β1 expression levels in the initial and recurrent groups.Enzyme-linked immunosorbent assay (ELISA) was used to detect IL-6 and TGF in the tissue homogenate of the initial,recurrent and control groups.-β1 concentration.Results The pathological grade of 24 patients with glioma increased significantly compared with the initial stage.The proportion of patients with grade III and IV in the relapse group was significantly higher than that in the initial group.The proportion of patients with grade I and grade II was significantly lower than that of the initial stage.The difference was statistically significant (P<0.05).Regulatory T cells were scattered and infiltrated in glioma tissues.With the increase of pathological grade,the positive rate of regulatory T cells was significantly increased (P<0.01).The positive rate of regulatory T cells in recurrent group was obvious.Compared with the initial group,the difference was statistically significant (P<0.01).IL-6 and TGF-β1 were clustered in glioma tissues.With the increase of pathological grade,the positive rates of IL-6 and TGF-β1 were significantly increased (P<0.01).The positive rate of IL-6 and TGF-β1 in the recurrent group was significantly higher than that in the initial group (P<0.05).The concentrations of IL-6 and TGF-β1 in the glioma tissue homogenate of the primary and recurrent groups were significantly higher than those of the control group.The concentrations of IL-6 and TGF-β1 in the recurrent group were significantly higher than those in the initial group.The difference was statistically significant.(P<0.01).Conclusion Regulatory T cell infiltration and expression of IL-6 and TGF-β1 in glioma microenvironment are increased,and the degree of malignancy and glioma recurrence are significantly increased.It can be used as an immune index to evaluate the progression and prognosis of glioma.
【Key words】 Glioma;Regulatory T cells;IL-6;TGF-β1;Expression changes;Relapse
胶质瘤是最常见的原发性颅内恶性肿瘤,具有恶性程度高、易复发、预后差的特点[1-8]。目前临床仍缺乏有效的治疗方案,免疫疗法作为近年来肿瘤领域研究的热点,为改善胶质瘤患者预后提供了新希望[9-15]。研究发现,免疫耐受和免疫逃逸在胶质瘤发生、发展中发挥重要作用[15-21]。调节性T细胞是表达CD4、CD25、FOXP3的免疫负调节T细胞,参与免疫耐受和免疫逃逸的调节过程[22-28]。 IL-6、TGF-β1为细胞免疫抑制因子,在肿瘤微环境中由癌细胞分泌,抑制体内免疫杀伤作用,促进癌细胞的增殖、迁移和转移[29-30]。本研究通过检测初发和复发胶质瘤组织中调节性T细胞、IL-6、TGF-β1的表达水平,探讨其与胶质瘤病理分级和病情复发的关系。
1 对象与方法
1.1 研究对象 选取2016-06-2017-10南阳市第二人民医院神经外科收治的胶质瘤患者24例,胶质瘤初发时及术后病情复发均在南阳市第二人民医院行手术治疗。排除标准:合并全身性炎症反应、自身免疫性疾病患者。男14例,女10例;年龄5~77(41.35±10.69)岁;复发间隔时间(11.04±3.61)个月。取患者2次术后组织标本共48份,分为初发组、复发组;根据世界卫生组织2016年颁布的中枢神经系统肿瘤分类与分级标准进行病理分级:Ⅰ级6例次,Ⅱ级14例次,Ⅲ级17例次,Ⅳ级11例次,其中Ⅰ级和Ⅱ级为低级别胶质瘤,Ⅲ级和Ⅳ级为高级别胶质瘤。另取同期脑外伤减压手术切除的正常脑组织标本6份为对照组。上述脑组织标本术中切除后,在1.5 h内分为2份,其中1份放入液氮中冻存,用于蛋白提取,另1份常规进行石蜡包埋。本研究经医院伦理委员会批准,患者或其家属签署知情同意书。
1.2 免疫组化染色 取脑组织石蜡包块,4 μm连续切片,脱蜡至水,3% H2O2消除内源性过氧化物酶,枸橼酸盐修复抗原30 min,室温下冷却,滴加正常山羊血清封闭10 min,分别加一抗:小鼠抗人IL-6单克隆抗体(1:500稀释)、兔抗人TGF-β1多克隆抗体(1:200稀释),4 ℃孵育过夜;滴加HRP标记的二抗,室温静置30 min,DAB显色、苏木素复染、脱水、封片。另取组织切片重复上述操作至正常山羊血清封闭10 min后,滴加一抗:兔抗人FOXP3单克隆抗体(1:100稀释)、小鼠抗人CD4细胞核抗体(1:100稀释)4 ℃孵育过夜;分别滴加磷酸酶及HRP标记的二抗,室温静置30 min,双染DAB显色,无菌蒸馏水终止反应,双染AP-Red再次显色,苏木素复染、脱水、封片。用磷酸缓冲液代替一抗作阴性对照,淋巴结组织切片作阳性对照。结果判定:调节性T细胞(CD4+ FOXP3+)阳性为细胞浆红色、细胞核棕黄色;IL-6、TGF-β1阳性为细胞浆棕黄色。光学显微镜高倍镜视野下随机选取10个视野进行观察、拍照,计算阳性细胞比例,以100个细胞中阳性细胞个数表示。
1.3 酶联免疫吸附试验检测 取胶质瘤组织和正常脑组织经组织匀浆后取上清液,BCA法进行蛋白定量,调整组织上清液中总蛋白含量相同,分别取100 μL上清液,使用IL-6、TGF-β1酶联免疫吸附试剂盒测定含量,严格按试剂盒说明进行操作。
1.4 统计学分析 采用SPSS 23.0分析,计量资料以均数±标准差(x±s)表示,多组间比较行F检验,2组间比较行t检验,计数资料以率(%)表示,进行χ2检验,P<0.05为差异有统计学意义。
2 结果
2.1 胶质瘤复发前后病理级别分布情况比较 24例胶质瘤患者病情复发后病理级别较初发时明显增加,其中复发组Ⅲ级和Ⅳ级患者比例明显高于初发组,Ⅰ级和Ⅱ级患者比例明显低于初发组,差异有统计学意义(P<0.05)。见表1。
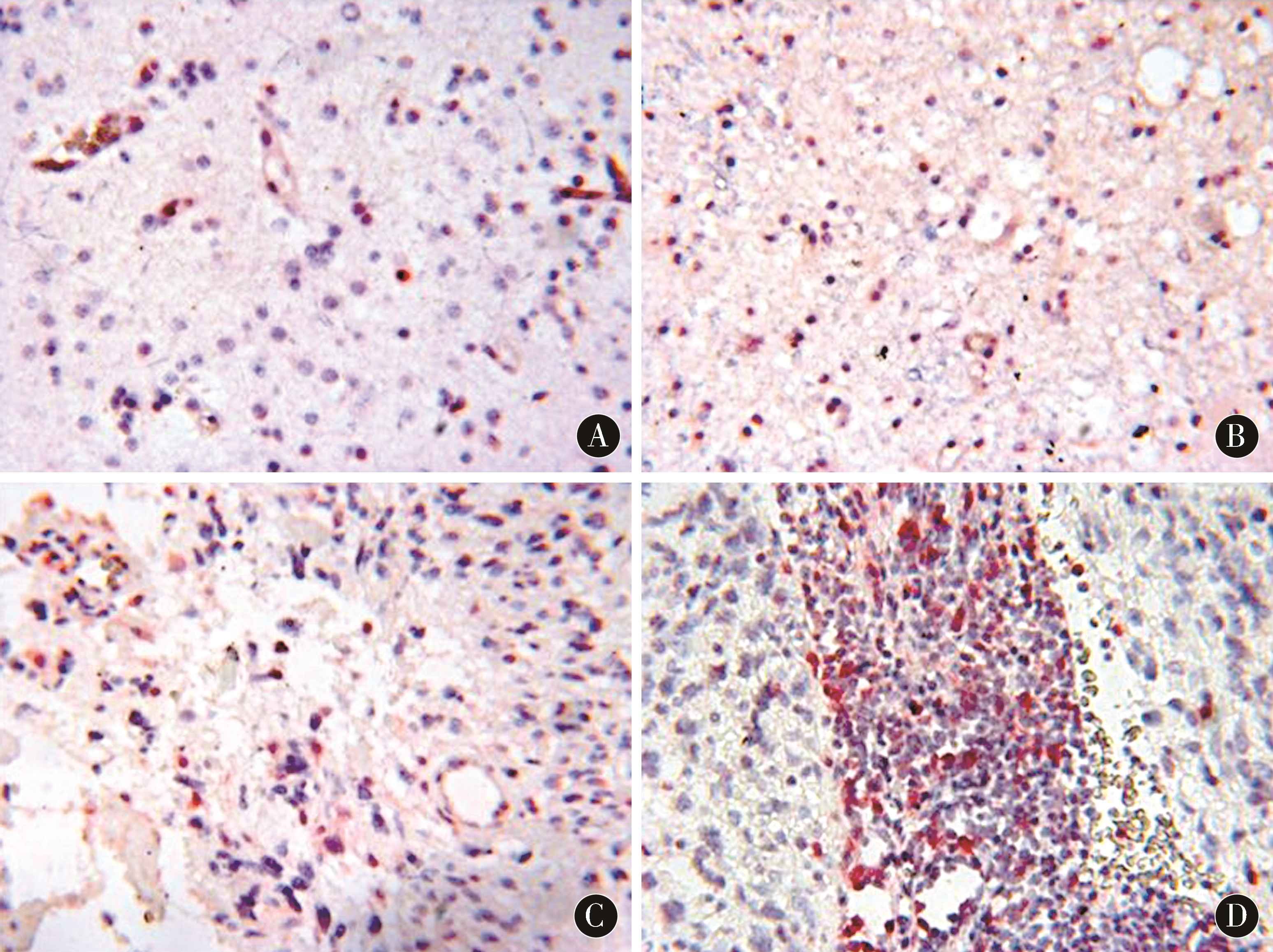
2.2 调节性T细胞在胶质瘤组织切片中的表达 调节性T细胞在胶质瘤组织中呈散在浸润,病理分级Ⅰ~Ⅳ级的调节性T细胞阳性率分别为(2.48±0.51)%、(4.37±1.42)%、(7.69±1.13)%、(10.93±2.50)%,随病理分级的增加,调节性T细胞阳性率明显提升,差异有统计学意义(P<0.01)。其中复发组调节性T细胞阳性率为(8.81±1.75)%,明显高于初发组的(5.38±1.42)%,差异有统计学意义(P<0.05)。见图1。
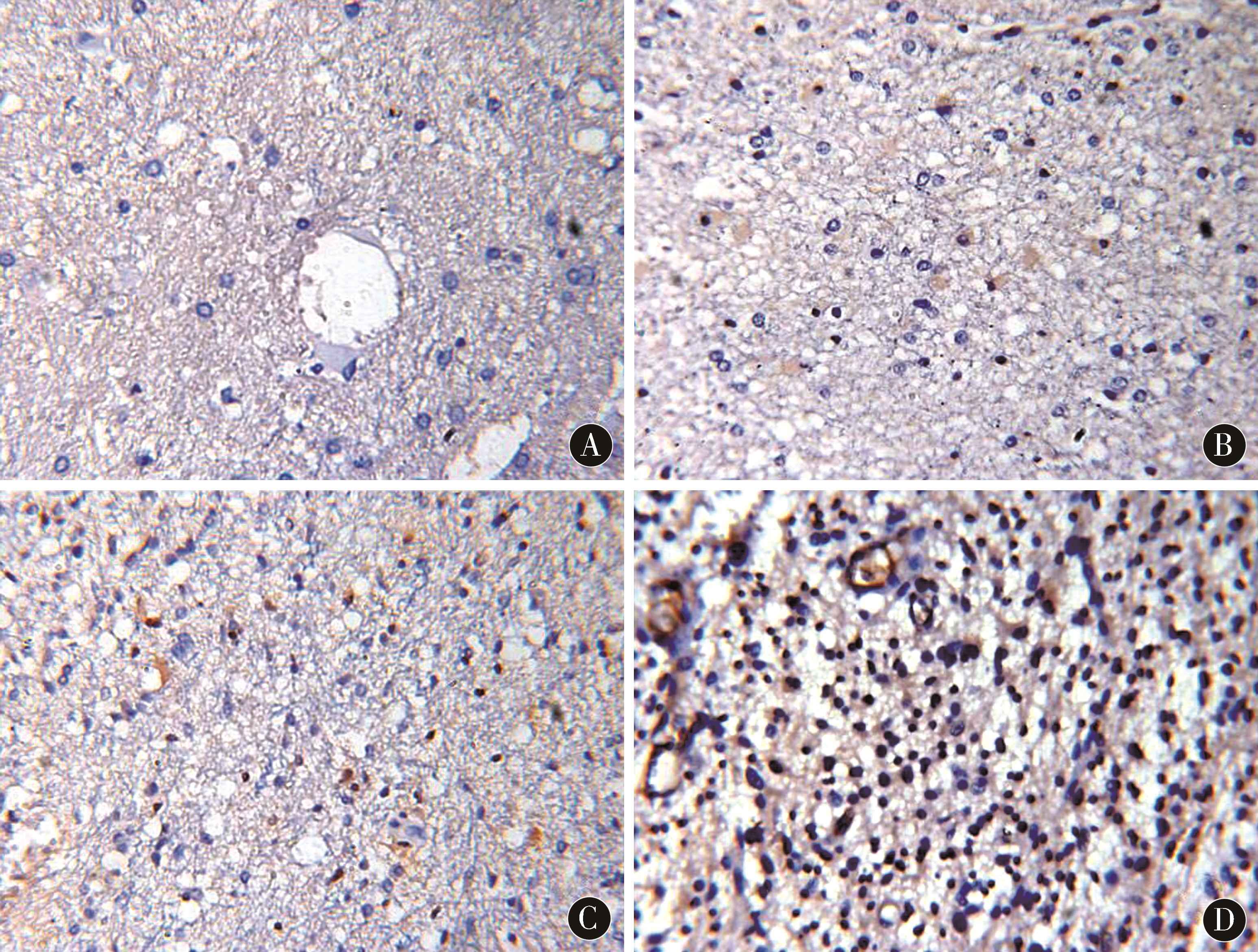
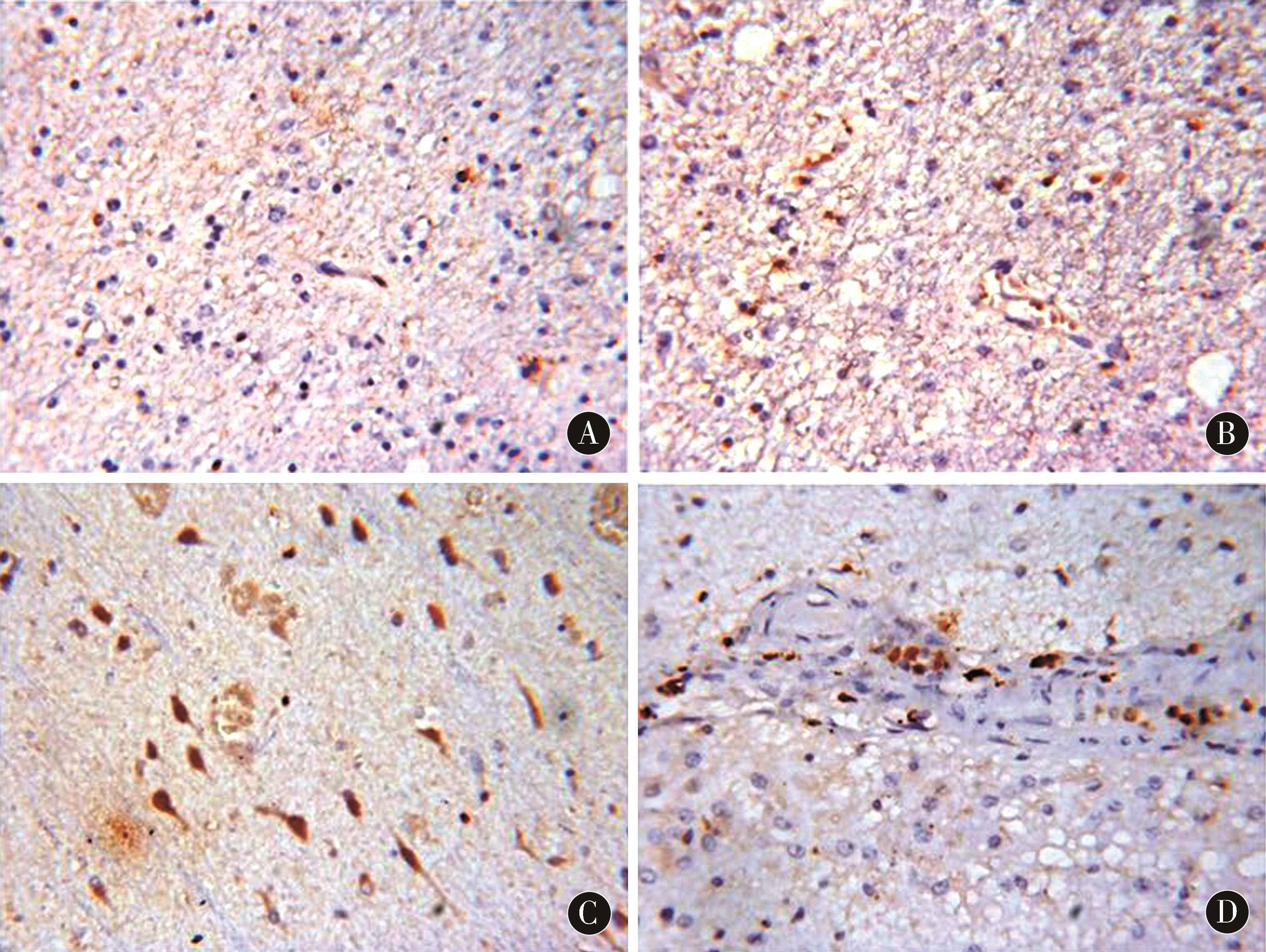
2.3 IL-6、TGF-β1在胶质瘤组织切片中的表达情况IL-6、TGF-β1在胶质瘤组织中呈团簇状分布,病理分级Ⅰ~Ⅳ级的IL-6阳性率分别为(7.24±1.42)%、(13.90±1.85)%、(19.71±2.13)%、(25.35±2.43)%;TGF-β1阳性率分别为(3.51±0.64)%、(6.94±0.79)%、(8.55±0.93)%、(12.07±1.42)%。随病理分级的增加,IL-6、TGF-β1阳性率均明显提升,差异有统计学意义(P<0.01)。其中复发组IL-6、TGF-β1阳性率明显高于初发组,差异有统计学意义(P<0.05)。见图2、图3、表2。
2.4 各组脑组织匀浆中IL-6、TGF-β1浓度比较 初发组与复发组的胶质瘤组织匀浆中IL-6、TGF-β1浓度明显高于对照组,复发组IL-6、TGF-β1浓度明显高于初发组,差异有统计学意义(P<0.01)。见表3。
表1 胶质瘤复发前后病理级别分布情况比较 [n(%)]
Table 1 Comparison of pathological grade distribution of glioma before and after recurrence [n(%)]
| 组别 |
n |
Ⅰ级 |
Ⅱ级 |
Ⅲ级 |
Ⅳ级 |
| 初发组 |
24 |
6(25.00) |
9(37.50) |
6(25.00) |
3(12.50) |
| 复发组 |
24 |
0(0.00) a |
5(20.83) a |
11(45.83) a |
8(33.34) a |
注:与初发组相比,a P<0.05
表2 初发组与复发组IL-6、TGF-β1表达阳性率比较 (%)
Table 2 Comparison of the positive rates of IL-6 and TGF-beta 1 expression between the initial group and the relapse group (%)
| 组别 |
n |
IL-6 |
TGF-β1 |
| 初发组 |
24 |
16.39±3.91 |
7.61±0.95 |
| 复发组 |
24 |
27.84±5.36 a |
12.97±1.60 a |
注:与初发组相比,aP<0.05
图1 调节性T细胞在胶质瘤组织中的表达情况(DAB、AP-Red染色,×400) A:Ⅰ级胶质瘤;B:Ⅱ级胶质瘤;C:Ⅲ级胶质瘤;D:Ⅳ级胶质瘤
Figure 1 The expression of regulatory T cells in glioma tissues (DAB,AP-Red staining,×400) A:Grade I glioma;B:Grade II glioma;C:Grade III glioma;D:Grade IV glioma
图2 IL-6在胶质瘤组织中的表达情况(DAB染色,×400) A:Ⅰ级胶质瘤;B:Ⅱ级胶质瘤;C:Ⅲ级胶质瘤;D:Ⅳ级胶质瘤
Figure 2 The expression of IL-6 in glioma tissue (DAB staining,×400) A:Grade I glioma;B:Grade II glioma;C:Grade III glioma;D:Grade IV glioma
图3 TGF-β1在胶质瘤组织中的表达情况(DAB染色,×400) A:Ⅰ级胶质瘤;B:Ⅱ级胶质瘤;C:Ⅲ级胶质瘤;D:Ⅳ级胶质瘤
Figure 3 The expression of TGF-beta 1 in glioma tissue (DAB staining,×400) A:Grade I glioma;B:Grade II glioma;C:Grade III glioma;D:Grade IV glioma
表3 各组脑组织匀浆中IL-6、TGF-β1浓度比较 (x±s)
Table 3 Comparison of IL-6 and TGF-beta 1 in brain homogenate of each group (x±s)
| 组别 |
n |
IL-6(pg/mL) |
TGF-β1(ng/mL) |
| 对照组 |
6 |
2.38±0.11 |
1.14±0.06 |
| 初发组 |
24 |
42.16±18.29 a |
8.42±1.49 a |
| 复发组 |
24 |
87.92±26.15 ab |
14.29±2.83 ab |
注:与对照组相比,aP<0.01;与初发组相比,bP<0.01
3 讨论
胶质瘤是最常见的原发性中枢系统肿瘤,约占所有原发性颅内肿瘤的一半,具有恶性程度高、易复发的特点,患者5 a生存率低于10%。目前研究认为,免疫耐受和免疫逃逸是胶质瘤发生、发展的主要机制。调节性T细胞可通过多种炎症因子抑制树突状细胞成熟和抗原呈递,阻止杀伤性T细胞活化,促进免疫耐受和免疫逃逸。本研究显示,胶质瘤微环境中存在调节性T细胞高表达,且随病理分级的增加而提升;胶质瘤复发时的调节性T细胞水平明显高于初发时。提示调节性T细胞可作为预测胶质瘤恶变和复发的免疫指标。
研究发现,IL-6作为肿瘤生长因子的同时也是免疫抑制因子,下调IL-6表达后可有效抑制肿瘤细胞的迁移和侵袭。TGF-β1在肿瘤细胞迁移、侵袭过程中发挥重要作用,能够调节性T细胞的扩增和诱导生成,当TGF-β1被特异性抗体阻断后,调节性T细胞水平明显下降,机体免疫应答提升。相关研究显示,调节性T细胞在肿瘤微环境中浸润增加,IL-6、IL-10、TGF-β1等细胞因子在其中发挥辅助作用。本研究显示,胶质瘤微环境中存在IL-6、TGF-β1高表达,且随病理分级的增加而提升;胶质瘤复发时的IL-6、TGF-β1水平明显高于初发时。进一步比较正常脑组织与胶质瘤组织匀浆中调节性T细胞、IL-6、TGF-β1的浓度发现,胶质瘤组织匀浆IL-6、TGF-β浓度明显高于正常脑组织,且复发组浓度明显高于初发组,提示IL-6、TGF-β在胶质瘤发生、恶变、复发中发挥重要调节作用,可用于胶质瘤的免疫治疗和预后判断。
4 参考文献
[1] ZHANG B,WANG H,LIAO Z,et al.EGFP-EGF1-conjugated nanoparticles for targeting both neovascular and glioma cells in therapy of brain glioma[J].Biomaterials,2014,35(13):4 133-4 145.
[2] ZHANG B,SHEN S,LIAO Z,et al.Targeting fibronectins of glioma extracellular matrix by CLT1 peptide-conjugated nanoparticles[J].Biomaterials,2014,35(13):4 088-4 098.
[3] GAO H,YANG Z,ZHANG S,et al.Glioma-homing peptide with a cell-penetrating effect for targeting delivery with enhanced glioma localization,penetration and suppression of glioma growth[J].J Control Release,2013,172(3):921-928.
[4] GIERYNG A,PSZCZOLKOWSKA D,BOCIAN K,et al.Immune microenvironment of experimental rat C6 gliomas resembles human glioblastomas[J].Sci Rep,2017,7(1):17 556.
[5] JIANG X,XIN H,REN Q,et al.Nanoparticles of 2-deoxy-d-glucose functionalized poly(ethylene glycol)-co-poly(trimethylene carbonate) for dual-targeted drug delivery in glioma treatment[J].Biomaterials,2014,35(1):518-529.
[6] CHEN L,MIAO W,TANG X,et al.The expression and significance of neuropilin-1 (NRP-1) on glioma cell lines and glioma tissues[J].J Biomed Nanotechnol,2013,9(4):559-563.
[7] MCFARLAND B C,MARKS M P,ROWSE A L,et al.Loss of SOCS3 in myeloid cells prolongs survival in a syngeneic model of glioma[J].Oncotarget,2016,7(15):20 621-20 635.
[8] DOMINGUES P,GONZÁLEZ-TABLAS M,OTERO Á,et al Tumor infiltrating immune cells in gliomas and meningiomas[J].Brain Behav Immun,2016,53:1-15.
[9] GIELEN P R,SCHULTE B M,KERS-REBEL E D,et al.Increase in both CD14-positive and CD15-positive myeloid-derived suppressor cell subpopulations in the blood of patients with glioma but predominance of CD15-positive myeloid-derived suppressor cells in glioma tissue[J].J Neuropathol Exp Neurol,2015,74(5):390-400.
[10] CHOI B D,GEDEON P C,HERNDON J E 2ed,et al.Human regulatory T cells kill tumor cells through granzyme-dependent cytotoxicity upon retargeting with a bispecific antibody[J].Cancer Immunol Res,2013,1(3):163.
[11] CHENG M W,WANG B C,WENG Z Q.et al.Clinicopathological significance of Polo-like kinase 1 (PLK1) expression in human malignant glioma[J].Acta Histochem,2012,114(5):503-509.
[12] MAES W,VERSCHUERE T,VAN HOYLANDT A,et al.Depletion of regulatory T cells in a mouse experimental glioma model through anti-CD25 treatment Results in the infiltration of non-immunosuppressive myeloid cells in the brain[J].Clin Dev Immunol,2013,2013:952 469.
[13] ZENG J,SEE A P,PHALLEN J,et al.Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas[J].Int J Radiat Oncol Biol Phys,2013,86(2):343-349.
[14] LOHR J,RATLIFF T,HUPPERTZ A,et al.Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-β[J].Clin Cancer Res,2011,17(13):4 296-4 308.
[15] SMITHA K A,GUPTA A K,JAYASREE R S.Total magnitude of diffusion tensor imaging as an effective tool for the differentiation of glioma[J].Eur J Radiol,2013,82(5):857-861.
[16] ZABORONOK A,ISOBE T,YAMAMOTO T,et al.Proton beam irradiation stimulates migration and invasion of human U87 malignant glioma cells[J].J RADIAT RES,2014,55(2):283-287.
[17] KARUNANITHI S,SHARMA P,KUMAR A,et al.Can 18F-FDOPA PET/CT predict survival in patients with suspected recurrent glioma? A prospective study[J].EUR J RADIOL,2014,83(1):219-225.
[18] LIU G,SHEN H,MAO J,,et al.Transferrin Modified Graphene Oxide for Glioma-Targeted Drug Delivery:In Vitro and in Vivo Evaluations[J].ACS Appl Mater Interfaces,2013,5(15):6 909-6 914.
[19] MATHEWS M S,BLICKENSTAFF J W,SHIH E C,et al.Photochemical internalization of bleomycin for glioma treatment[J].J Biomed Opt,2012,17(5):058001.
[20] RONIOTIS A,MANIKIS G C,SAKKALIS V,et al.High-Grade Glioma Diffusive Modeling Using Statistical Tissue Information and Diffusion Tensors Extracted from Atlases[J].IEEE Trans Inf Technol Biomed,2012,16(2):255-263.
[21] LAWS E R,PARNEY I F,HUANG W,et al.Survival following surgery and prognostic factors for recently diagnosed malignant glioma:data from the Glioma Outcomes Project[J].J Neurosurg,2003,99:467-473.
[22] GAO J Q,LV Q,LI L M,et al.Glioma targeting and blood-brain barrier penetration bydual-targeting doxorubincin liposomes[J].Biomaterials,2013,34(22):5 628-5 639.
[23] SHI Z D,QIAN X M,LIU C Y,et al.SAspirin-/TMZ-coloaded microspheres exert synergistic antiglioma efficacy ia inhibition of β-catenin transactivation[J].CNS Neurosci Ther,2013,19(2):98-108.
[24] TERÉS S,LLADO V,HIGUERA M,et al.2-Hydroxyoleate,a nontoxic membrane binding anticancer drug,induces glioma celldifferentiation and autophagy[J].Proc Natl Acad Sci U S A,2x±s012,109(22):8 489-8 494.
[25] HEIMBERGER A B,ABOU-GHAZAL M,REINA-ORTIZ C,et al.Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas[J].Clin Cancer Res,2008,14(16):5 166-5 172.
[26] SONABEND A M,ROLLE C E,LESNIAK M S.The role of regulatory T cells in malignant glioma[J].Anticancer Res,2008,28(2B):1 143-1 150.
[27] GRAF M R,SAUER J T,MERCHANT R E.Tumor infiltration by myeloid suppressor cells in response to T cell activation in rat gliomas[J].J Neurooncol,2005,3(1):29-36.
[28] MUNRO D A D,HOHENSTEIN P,COATE T M,et al.Refuting the hypothesis that semaphorin-3f/neuropilin-2 exclude blood vessels from the cap mesenchyme in the developing kidney[J].Dev Dyn,2017,246(12):1 047-1 056.
[29] DONG X,GUO W,ZHANG S,et al.Elevated expres-sion of neuropilin-2 associated with unfavorable prognosis in hepatocellular carcinoma[J].Onco Targets Ther,2017,10:3 827-3 833.
[30] YUAN J,LIU L,HU Q,et al.Mathematical modeling of brain glioma growth using modified reaction-diffusion equation on brain MR images[J].Comput Biol Med,2013,43(12):2 007-2 013.
(收稿2018-12-21)
本文责编:张喜民
本文引用信息:王栋,白雪蕾,吴环立.胶质瘤中调节性T细胞浸润及IL-6 TGF-β1表达与病情复发的相关性[J].中国实用神经疾病杂志,2019,22(1):17-22.DOI:10.12083/SYSJ.2019.01.004
Reference information:WANG Dong,BAI Xuelei,WU Huanli.Correlation between regulatory T cell infiltration and IL-6 TGF-β1 expression in gliomas and relapse[J].Chinese Journal of Practical Nervous Diseases,2019,22(1):17-22.DOI:10.12083/SYSJ.2019.01.004